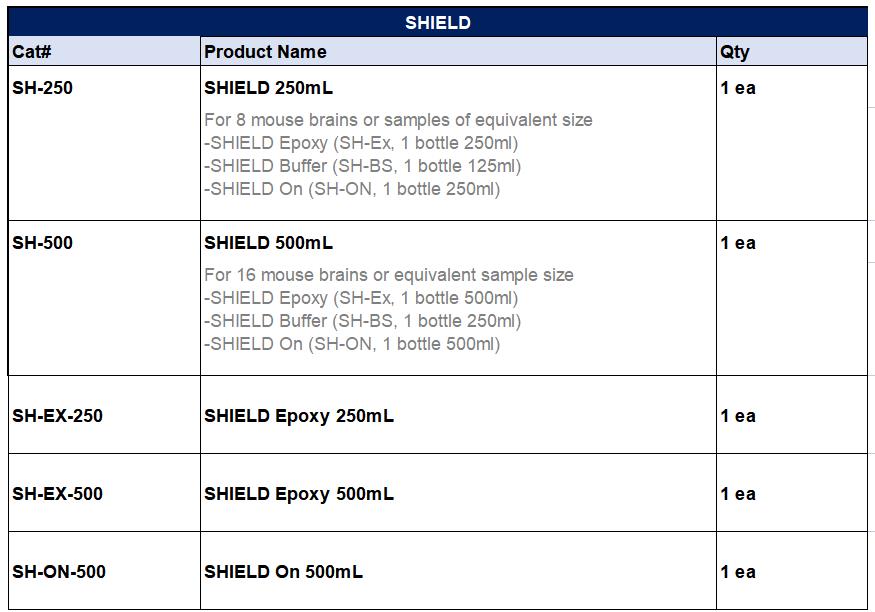



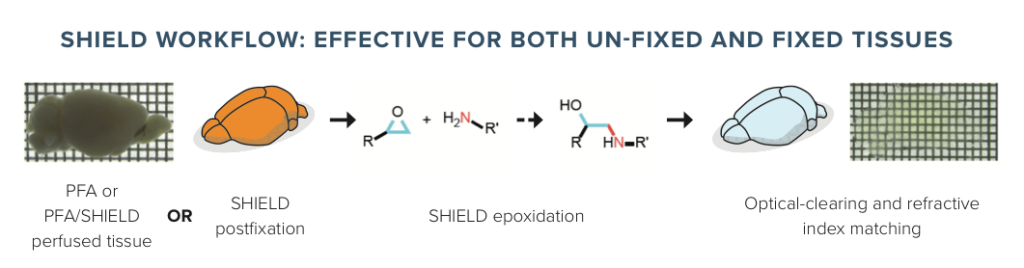

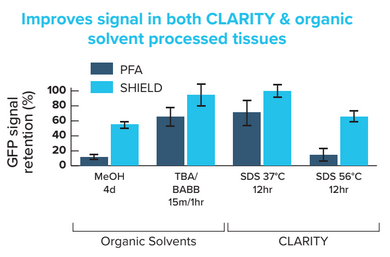
Standard Protocol:

Stopping Points: The protocol can only be stopped at the red circle points in the
timeline above, so plan ahead!
1. Transcardially perfuse the animal with ice-cold PBS. For mice, use about 20 mL and a 5
mL/min flow rate. For rats, use 200 mL and a 60 mL/min flow rate. We recommend using
heparinized PBS to remove as much blood as possible (20 U/mL concentration). Make
sure the fluid is running completely clear before next perfusing with ice-cold 4% PFA in
PBS. Use the same amounts and flow rates as before. Be careful not to introduce air
bubbles inside tubing. When the fluid comes out of the mouth or a lung swells, adjust the
position of the needle in the heart.
2. Dissect out the brain / organ of interest. Careful dissection is essential to preserve the
sample’s structural integrity.
3. Incubate the sample in 4% PFA in PBS for 24 hours at 4°C with shaking. If you are not
ready to continue to the next step, The sample can be stored in 1X PBS with 0.02%
sodium azide at 4°C until you are ready to continue.
4. Before proceeding, please check the Expiration Date on the SHIELD-Epoxy bottle. If the
solution is used after the expiration date the mechanical stability of the sample can be
compromised.
5. Prepare fresh SHIELD OFF Solution. Mix the following in the order shown in the table
and keep on ice or move to 4°C after mixing. After adding each reagent, please vortex or
mix well to prevent precipitate formation.
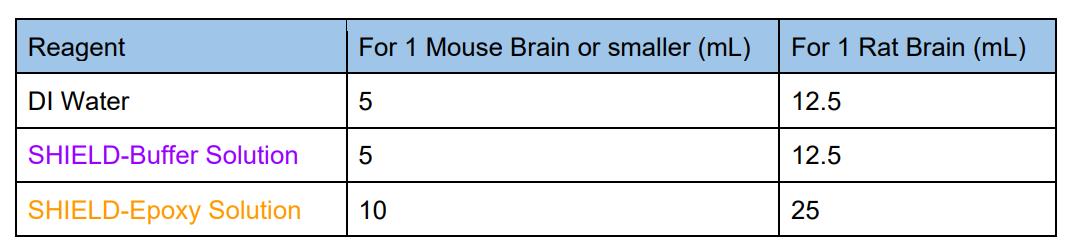
6. Incubate the sample in SHIELD OFF Solution at 4°C with shaking for 3 days for mouse
brains or similar sized samples. Rat brains should be incubated for 6 days. If your
sample’s smallest dimension is 1.5 mm or smaller, please stop here after incubation and
continue to the Small Sample SHIELD-ON protocol.
7. Pre-warm SHIELD ON Buffer to 37°C. For mouse brains or similar sized samples use 20
mL. Rat brains require 40 mL.
8. Transfer the sample to SHIELD ON Buffer (RT) and incubate at 37°C with shaking for 24
hours.
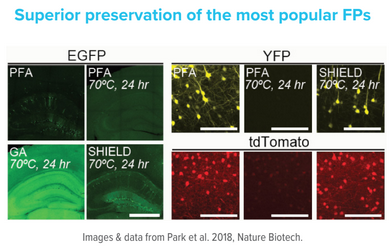
SHIELD preservation is now complete. The sample can be stored in 1X PBS with 0.02%
sodium azide at 4°C for several months without significant loss of fluorescence signal
and structural integrity.
9. You may now proceed to the tissue clearing section of the protocol.
Specifications: