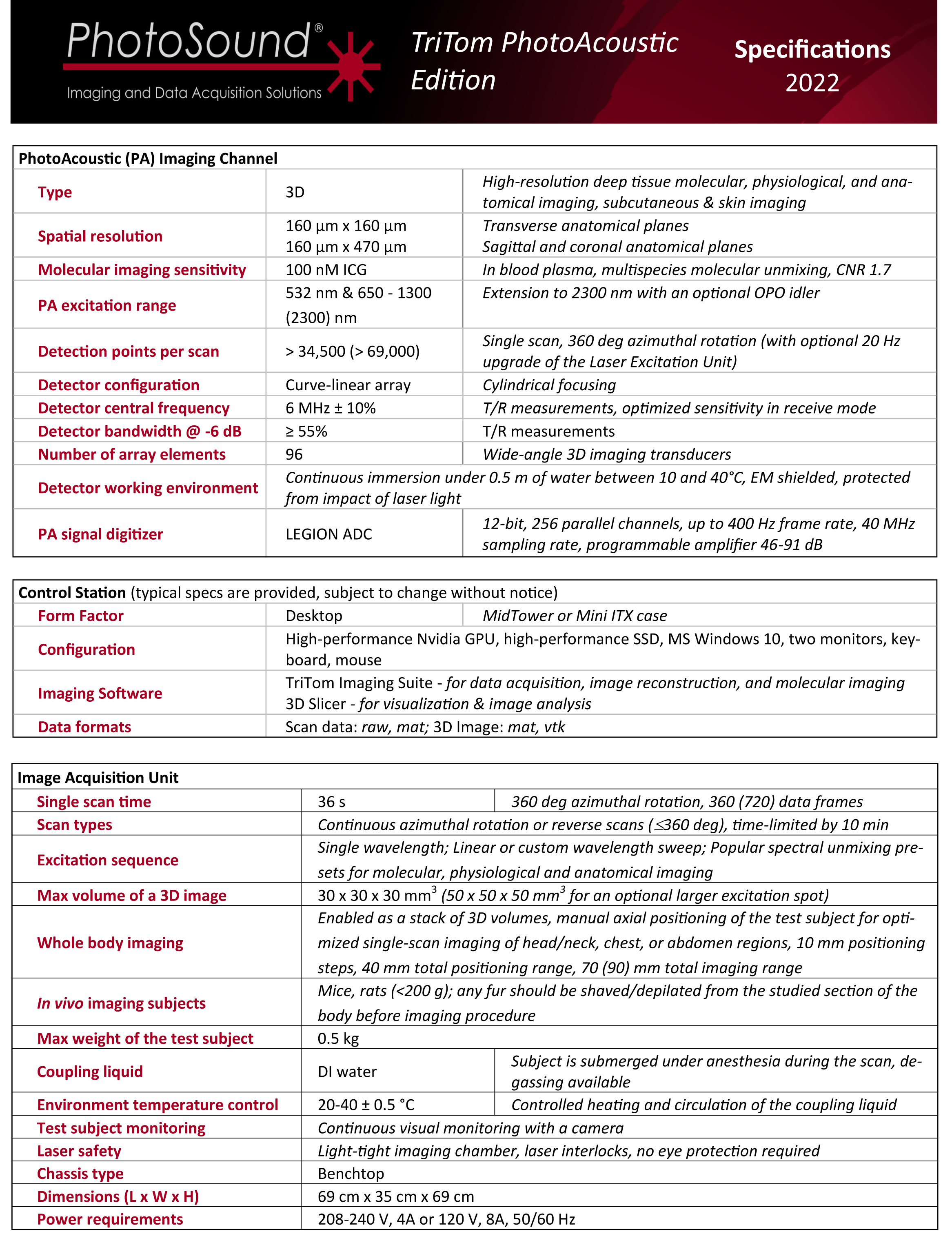
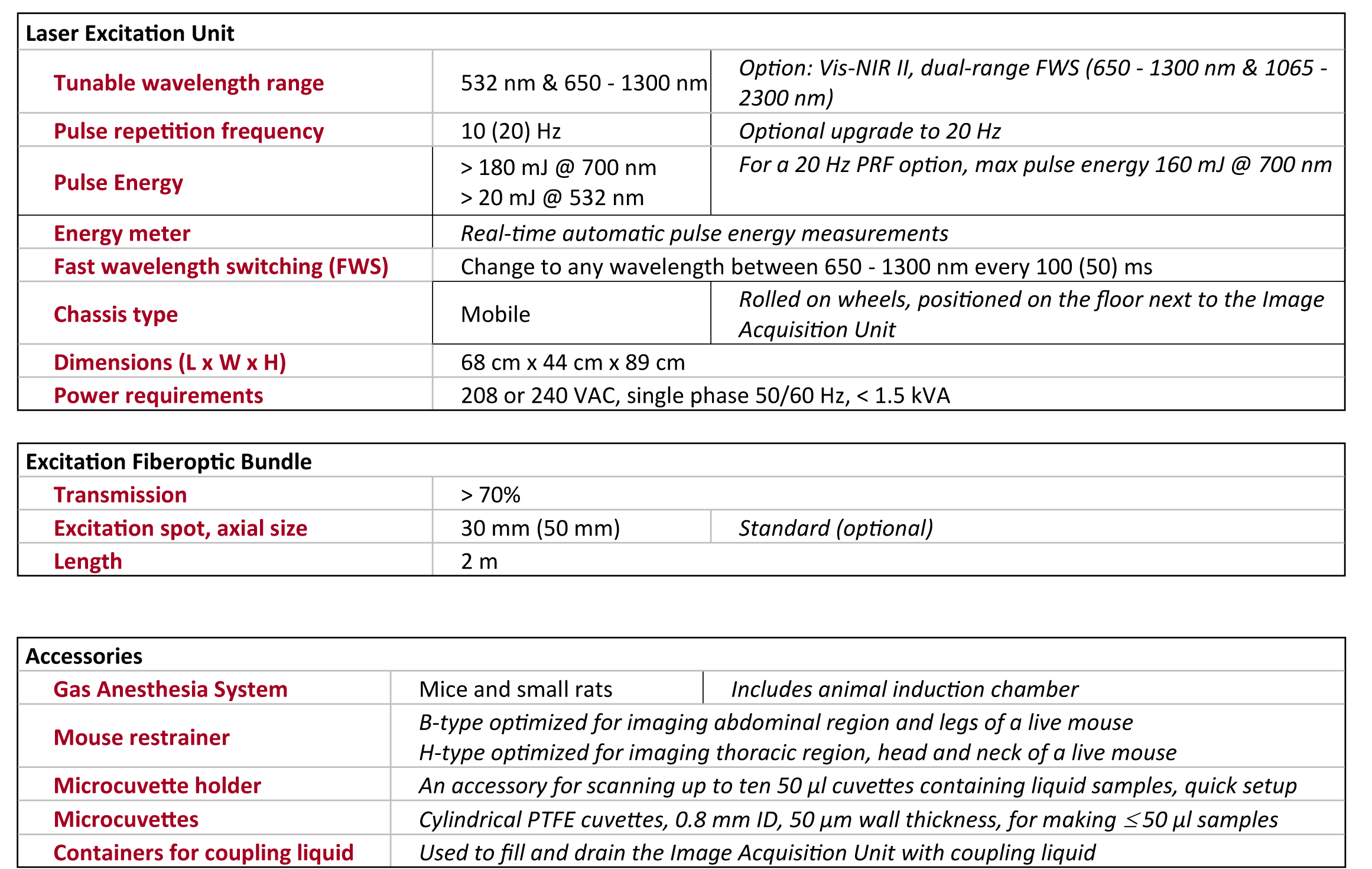
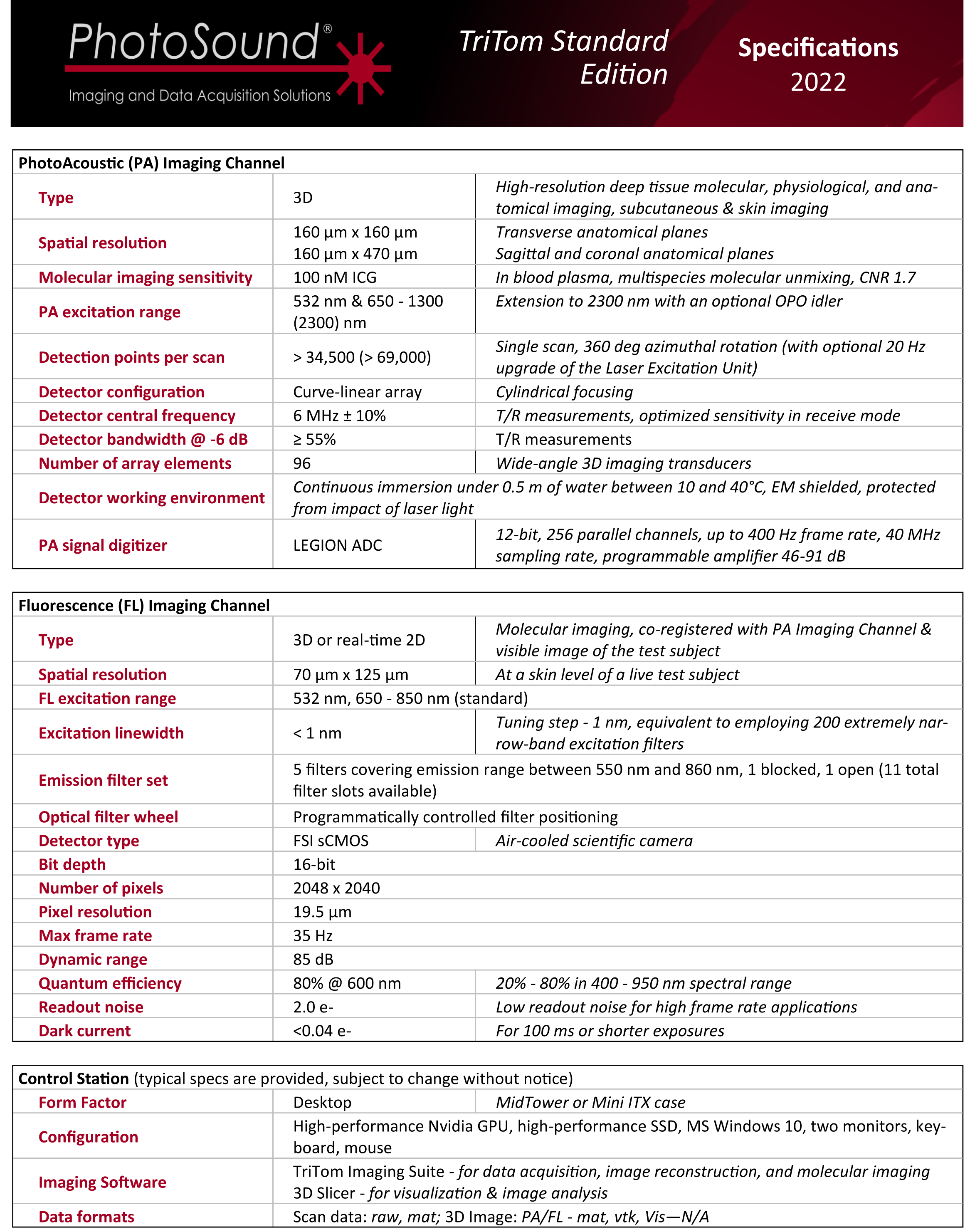
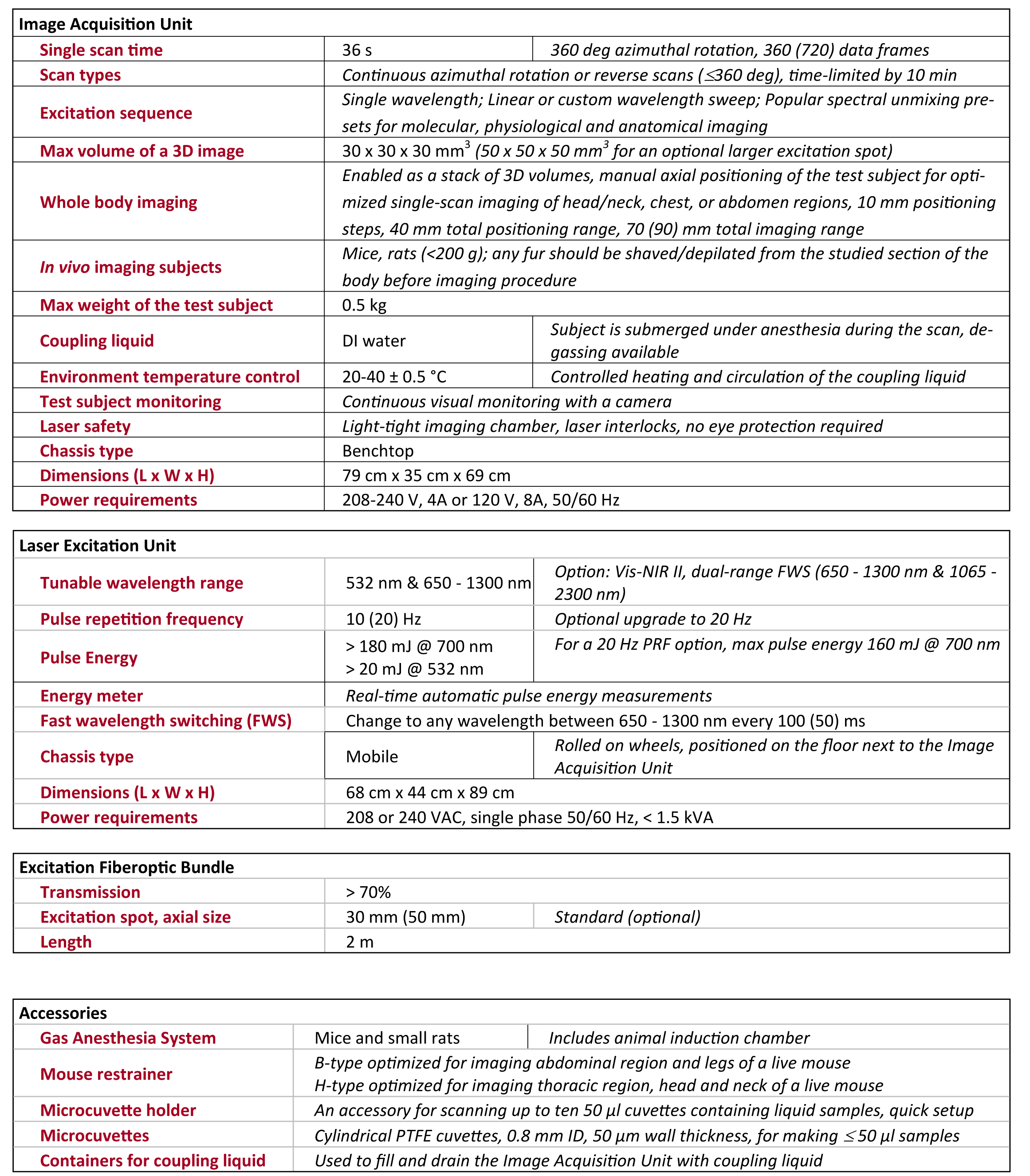
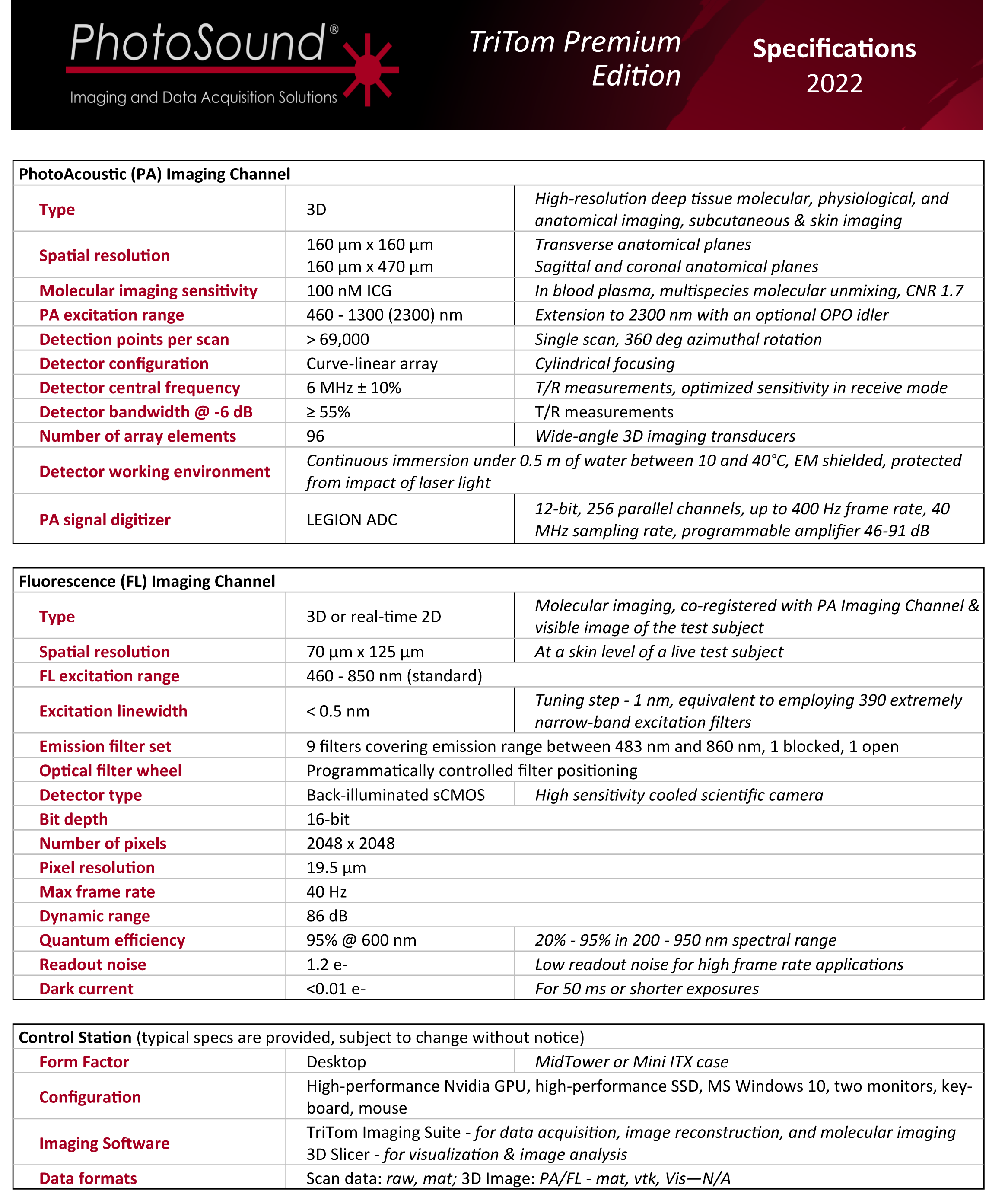
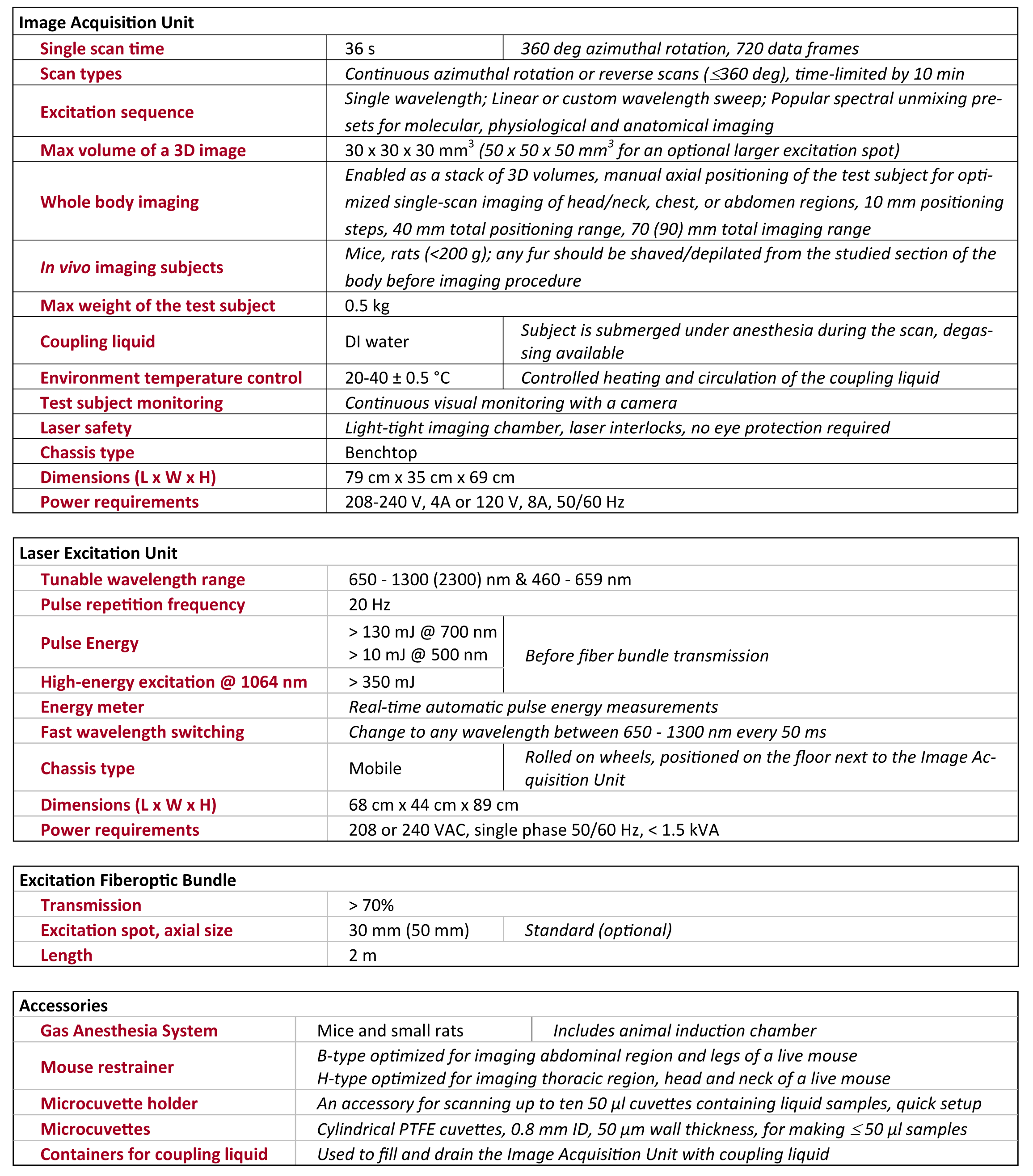
1. Anatomical Brain Imaging
Abstract: The imaging of vasculature in and around a mouse’s brain is essential for BLANK. A post-mortem BALC/c mouse with metastasized 4T1-luc is scanned using photoacoustic imaging (PAI) with the PhotoSound TriTom imaging platform. PAI is a non-invasive imaging method that produces high resolution volumetric data of tissue and vasculature. The head of the mouse is imaged at wavelengths of 750 nm and 532 nm
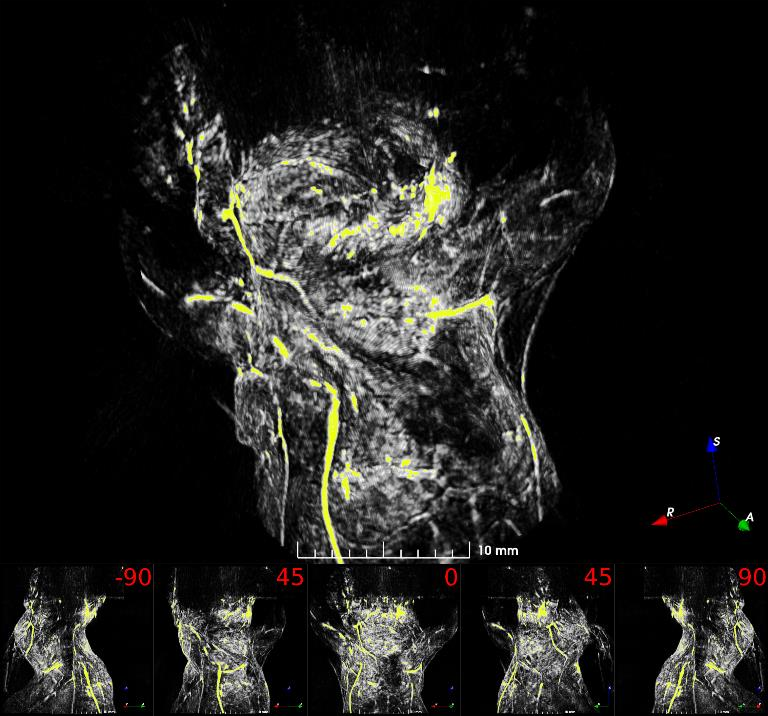
Fig. 1: Maximum intensity projection volume render of the scanned mouse at 532 nm. High intensity signals are visualized in yellow. Angle of rotation is displayed in the top-right corner of each view.
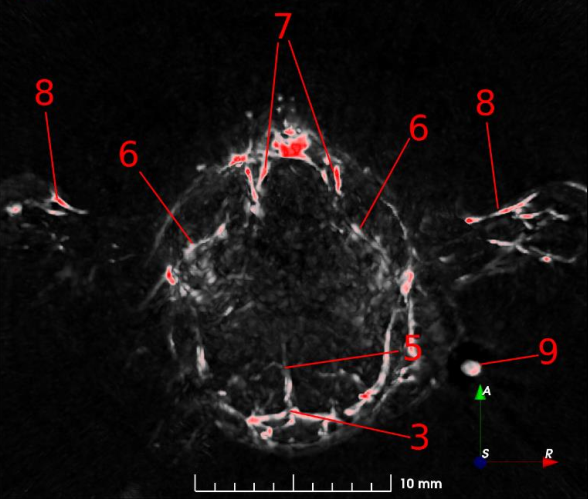
Fig. 2: 7 mm maximum intensity projection (MIP) slabs of 750 nm excitation PA reconstruction volumes. A: Dorsoventral view of the mouse’s scalp. B: Transverse view of mouse’s brain near the cerebellum/medulla. (1) transverse sinus; (2) superior sagittal sinus; (3) confluence of sinus; (4) auricular artery; (5) cerebral artery; (6) ophthalmic artery; (7) jugular vein; (8) brachial artery; (9) CuSO4 tube.
 Application Note 1.pdf
Application Note 1.pdf
2. Whole Body Anatomical Imaging
Noninvasive whole-body imaging of small animals is crucial for understanding the fundamental relationship between anatomical structure and function. Optical methods have a high spatial resolution but suffer from shallow penetration depth (1-2 mm). Nonoptical techniques such as MRI and PET provide the penetration depth but are costly, have long acquisition times, use ionizing radiation, or require exogenous contrast agents. Photoacoustic imaging takes advantage of the intrinsic optical properties of tissue, specifically hemoglobin, to provide high-resolution images of both superficial and deep blood-rich structures in murine models.
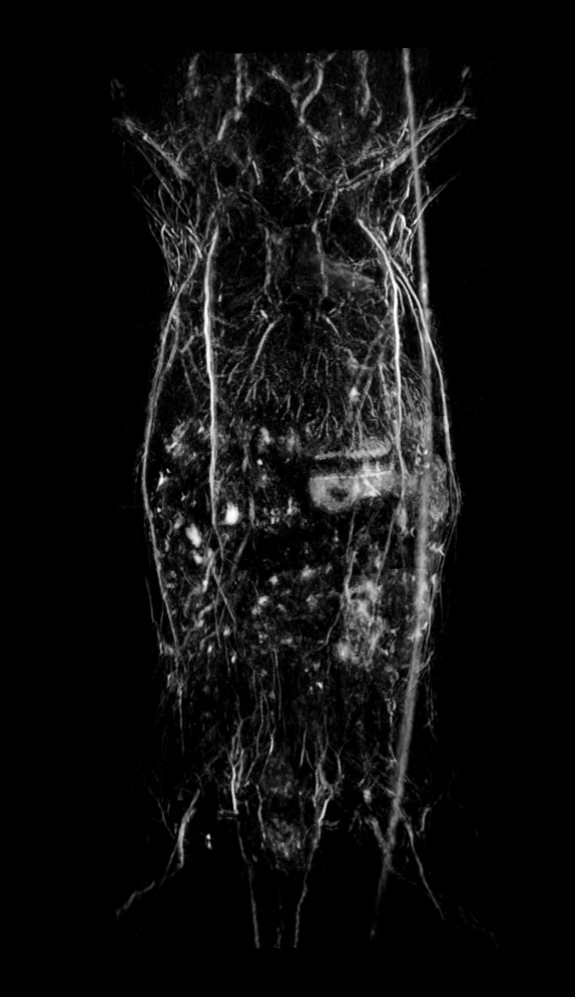
Fig.3 Whole-body anatomical PAT scan of a nu/nu nude mouse acquired at 800 nm laser excitation with the TriTom.
3. Functional Imaging
In addition to whole-body anatomical imaging, photoacoustic imaging can be utilized for functional imaging of physiological processes, which can include monitoring blood oxygenation, tumor growth, or therapeutic effects. Contrast agents, such as ICG, nanoparticles, etc., can be used to evaluate either blood-flow dynamics or for targeted imaging of vascular biomarkers. Here, we show high-resolution images of vasculature in the kidneys (Fig.4) and liver (Fig.5), demonstrating the ability to detect pathophysiological abnormalities in superficial and deep tissues.
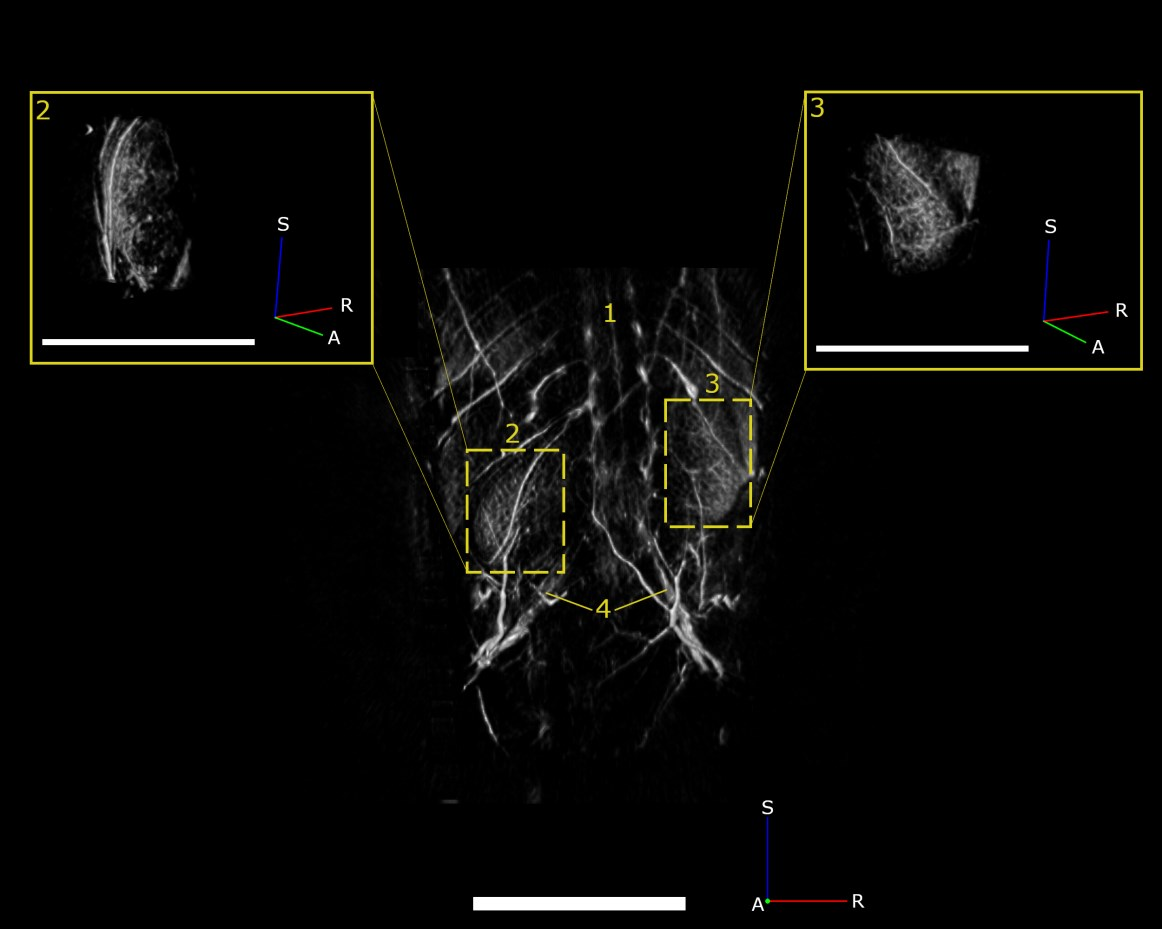
Fig.4 PAT maximum intensity projection of the spine (1), left kidney (2), right kidney (3), and iliac arteries (4) acquired at 750 nm in a female nu/nu nude mouse. Scale bar = 5mm.
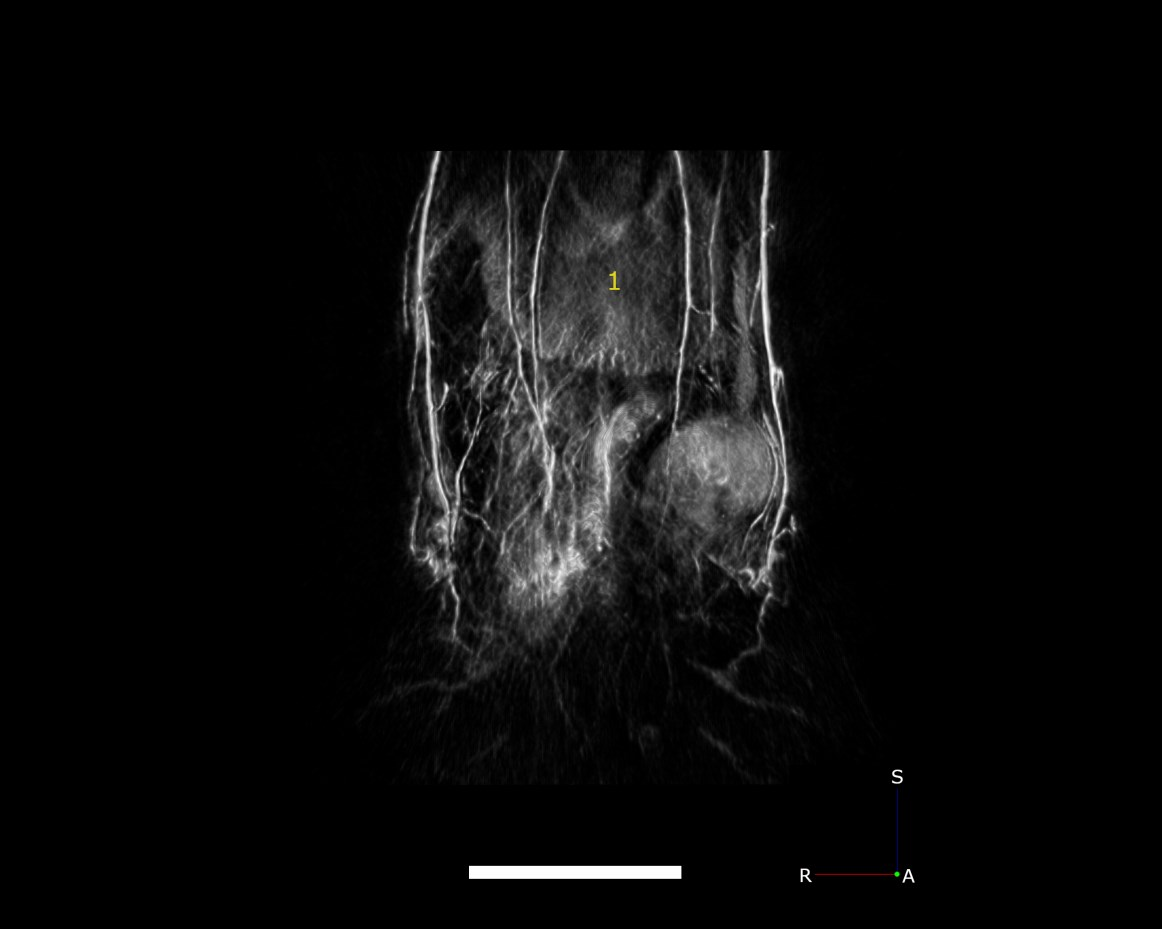
Fig.5 PAT maximum intensity projection of the mouse liver collected with 750 nm laser excitation. ale bar = 5mm.
 Application Note 2&3.pdf
Application Note 2&3.pdf
4. Imaging Mouse Spine
Abstract: Finding non-invasive, in vivo methods for imaging the spine is of great importance to improving surgery techniques and monitoring spinal injuries without causing further neurological damage. Photoacoustic imaging (PAI) has proven to be a low cost, safe, and highly informative modality for imaging the spine due to its ability to scan through bone tissue without using ionizing radiation. In this application note, the spine of a post-mortem, nu/nu nude mouse was scanned using the PhotoSound TriTom at a laser wavelength of 850 nm. Features of the vertebral body and spinal column are labeled in PAI images and compared to similar MRI anatomical images.
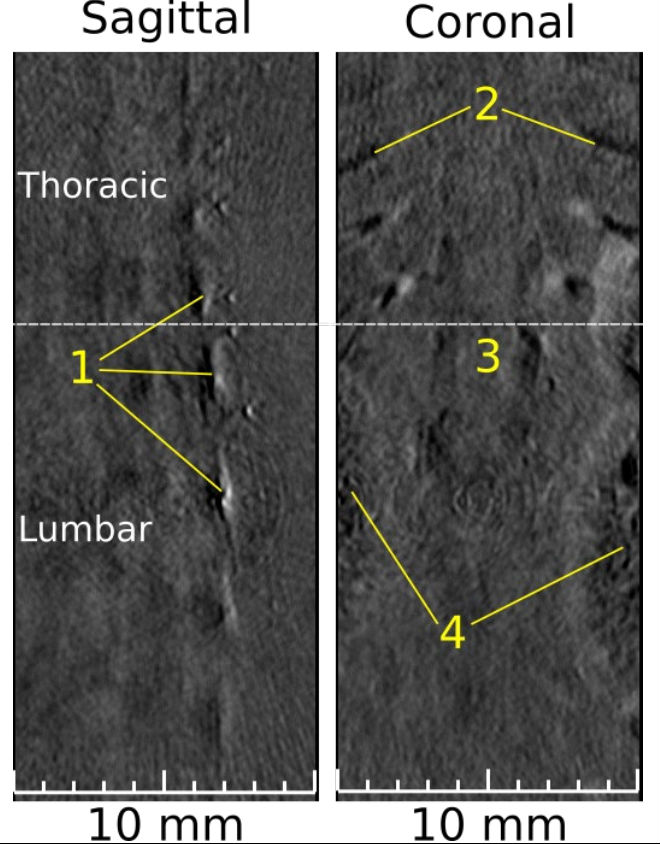
Fig. 6: PAT 2D slices, sagittal and coronal views, excited by 850 nm. (1) Multiple thoracic and lumbar vertebrae; (2) ribs; (3) grey/white matter; (4) kidneys. Thoracic and lumbar sections are separated by the dotted-white line.
 Application Note 4.pdf
Application Note 4.pdf
5. Imaging Tumor Xenograft
Abstract: an important step in understanding cancer metastasis in humans is first studying the disease in mice. By infecting a mouse using a subcutaneous injection of the BT-474 cell line, a human breast carcinoma, the resulting metastatic tumor’s growth can be tracked using photoacoustic imaging (PAI). PAI is a non-invasive imaging modality that can generate volumetric data that details the depth and dimensions of anatomical objects like tumors. An old, 2 years, Nu/Nu nude mouse is infected with a BT-474 xenograft and imaged in vivo using PAI with the PhotoSound TriTom system at a laser wavelength of 800 nm and 532 nm.
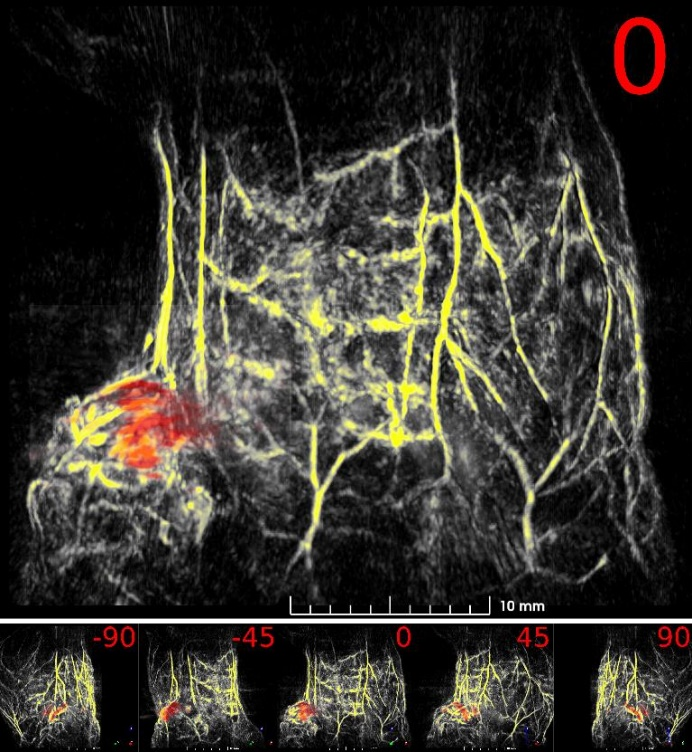
Fig. 7: Maximum intensity projection volume render of the scanned mouse. 532 nm volume is colored yellow, 800 nm tumor volume is colored red. Angle of rotation is displayed in the top-right corner of each view.
 Application Note 5.pdf
Application Note 5.pdf
6. Imaging Lymphatic Drainage
Abstract: The tracking of a contrast agent’s lymphatic drainage enables further case studies of metastasis progression in lymph nodes, which is crucial for evaluating cancer staging and prognosis of patients. Using a healthy nu/nu nude mouse, a 50 μl 1:1 mixture of glycol-chitosan coated gold nanoparticles (GC-AuNPs) and indocyanine green (ICG), both of concentration 50 μg/ml, was injected subcutaneously into the mammary right fat pad. Approximately 17 hours after injection, the mouse was photoacoustically (PA) scanned using a TriTom system. The results show that the injected GC-AuNPs/ICG contrast has drained from the mammary fat pad into the right subiliac lymph node.
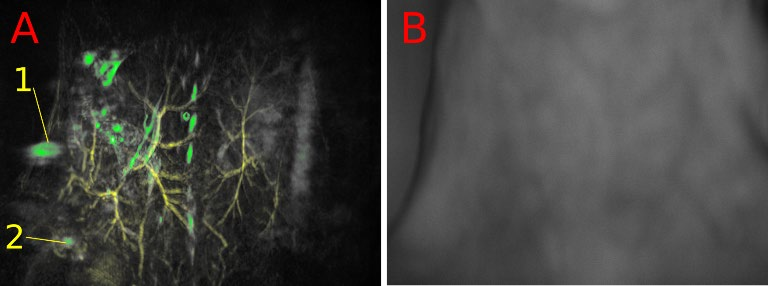
Fig 8: PAT volume (A) of the mouse’s skin (532 nm, yellow) and high PA amplitude objects (770 nm, green). The RSLN (1) and injection site (2) are marked. Optical image for anatomical reference (B).
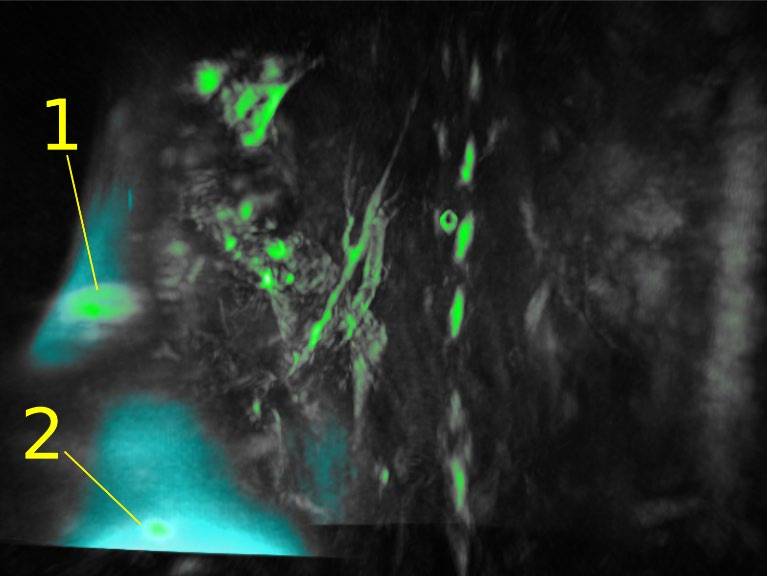
Fig 9: FL volume (770 nm, blue) and high PA amplitude objects (770 nm, green). The RSLN (1) and injection site (2) are marked.
 Application Note 6.pdf
Application Note 6.pdf
7. Neuroimaging
Brain imaging, or neuroimaging, is used to understand the relationship between brain function and behavior and study the underlying causes of neurological and psychiatric diseases. It is important in helping to understand the relationship between specific areas of the brain and what function they serve. However, there is currently no single imaging method capable of accurately mapping the brain’s complex anatomy and physiologic processes. Photoacoustic tomography (PAT) is a noninvasive and nonionizing imaging modality that can be used as a complementary tool to conventional imaging techniques used in preclinical neuroscience research such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET). PAT is capable of acquiring high contrast and resolution images of optical absorption with great depths.

Fig.10 PAT composite image of the upper torso and brain of a female BALB/c mouse acquired post-mortem with 532 nm excitation (orange) and 750 nm (grey).
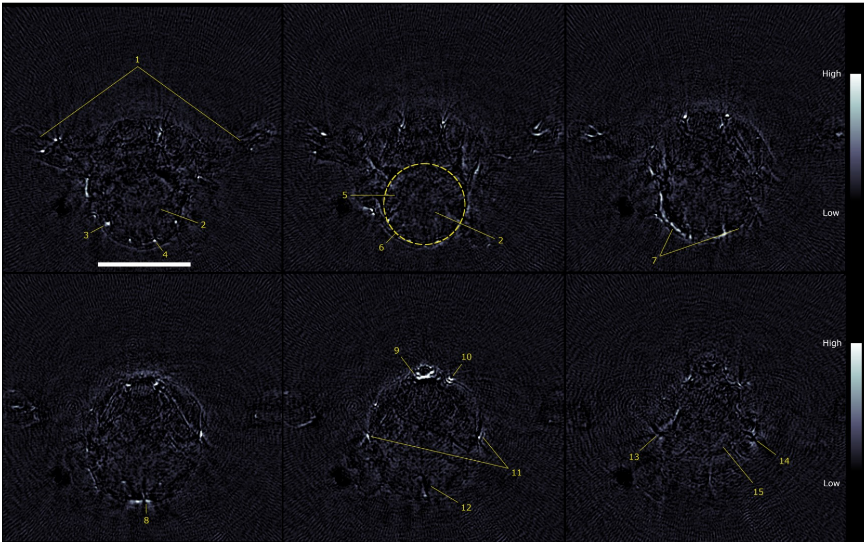
Fig.11 2D transverse slices of the 750 nm PAT scans acquired at several vertical displacements. (1) arms, (2) cerebellum, (3) auricular artery, (4) cerebral artery, (5) medulla, (6) outline of brain, (7) transverse sinus, (8) confluence of sinus, (9) sublingual vein, (10) facial vein, (11) superficial temporal vein, (12) subarachnoid space, (13) right eye, (14) left eye, (15) optic track. Scale bar = 5 mm.
 Application Note 7.pdf
Application Note 7.pdf