Go beyond traditional wound healing assays with the Phasefocus Livecyte
Directly measure cell motility
Separate cell motility from cell proliferation
Segment and track individual cells along the leading edge
Characterise morphological and behavioural cell phenotypes during wound healing
Introduction
The Livecyte system can be used to image the cells and analyse their behaviour as both an overall population and as single individual cells. The non-invasive nature of the measurement (label-free, low illumination power) ensures that the cells are unperturbed and behave in a more natural manner over an extended period of time. Very large field of views are available without stitching, which allows for statistically significant population metrics to be extracted (gap closure, proliferation), while the quantitative, high contrast nature of the images allows for single cell behaviour to be characterised (morphological parameters and motility). The Livecyte system generates a far greater suite of information from such established assays, compared to traditional methods such as brightfield, fluorescence or phase contrast.
Methods
MDA MB231 cells were seeded at 5x105 cells/ml in Ibidi culture-insert 2 well dishes (Cat. 81176) to create a cell free gap by exclusion. 24 hours after seeding the insert was removed and the cells were treated either with a vehicle control (1% BSA\HBSS) or with 50μM Lysophosphatidic acid (LPA). The Phasefocus Livecyte system was used to perform time-lapse imaging of the wound, every 10 minutes for a total period of 48 hours. The Phasefocus CAT (Cell Analysis Toolbox) software was used to segment and measure wound area, from which t1/2 gap (time for area of wound to reduce by half) and Vmigration (cell migration rate) was calculated. In addition, individual cells were segmented and tracked to report motility and morphological data.
The wound healing assay is an established in vitro procedure that measures collective cell migration.
The basis of this assay is to create a cell free region within a confluent layer of cells, either through the creation of a scratch by removing the cells post adherence, or through physical exclusion of cells during seeding. Once the cell free area has been created the closure of the wound is monitored through live-cell imaging. The measure of collective cell migration in this manner, replicates sheet migration that occurs in
a range of biological processes such as; cancer metastasis, embryonic morphogenesis and tissue injury.
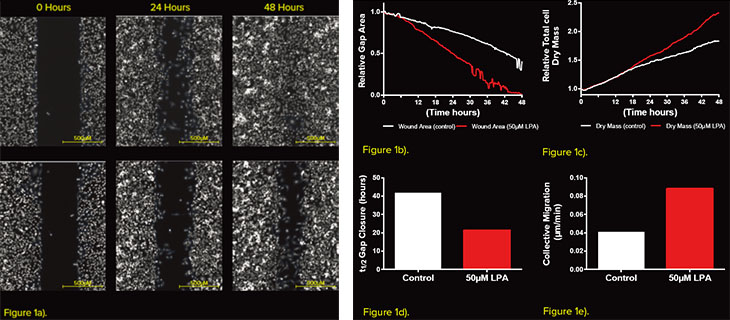
Figure 1 - Measuring Gap closure using the Phasefocus CAT software a) Representative images of wound gap segmented using the CAT software (blue outline), at 0, 24 and 48 hours and for both control and LPA treated cells. b) Relative gap area and c) relative dry mass measured throughout the time-lapse sequence. d) t1/2 gap closure and e) collective migration calculated from the GAP closure.
Results
The rate of gap closure is the most common metric that is measured from a wound healing assay. Subsequently, the t1/2 of the gap and Vmigration can be calculated. The Livecyte system was utilised to accurately determine the change in gap area over a 48-hour period (Fig. 1a). The addition of 50μM LPA caused the wound to close at a faster rate (Fig. 1a, b) with a t1/2 gap of 21.4 hours and Vmigration of 5.3μM/hour when compared to the control (41.9 hours and 2.5μM/hour, respectively. Fig. 1 d, e.) However, the change in gap area can be influenced by both cell migration and cell proliferation. It is therefore important to account for any changes induced as a result of cell proliferation. The quantitative nature of images acquired allows for the determination of optical volume of cells. Measuring total optical volume of all cells over time, gives an indication of the rate of cell growth and proliferation. In this experiment cells treated with 50μM LPA increased the rate of cell proliferation after 16 hours (Fig 1c). This indicates that post 16 hours both cell migration and proliferation had an influence on t1/2 gap and Vmigration. In addition to modelling collective migration, the additional capabilities of the Livecyte system allow for direct measurement of single cells at the leading edge of the wound (Fig. 2a). Tracking such cells revealed an increase in mean cell speed and instantaneous cell speed following application of 50μM LPA. The LPA treatment also induced a reduction in the meandering index, revealing that the behaviour of migration was also influenced (Fig. 2b). Further to motility parameters the virtue of individual cell segmentation, allowed for quantification of morphological parameters such as cell area, thickness, sphericity and length/width ratio. Treatment with 50μM LPA caused an increase in cell thickness and sphericity, whilst reducing the length/width ratio.
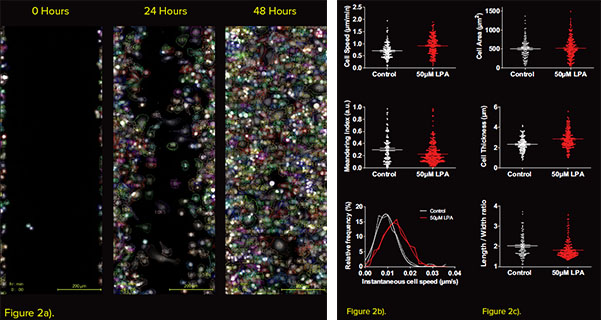
Figure 2 - Advanced segmentation and tracking of single cells at the leading edge a) Representative images of cells at the leading edge of the wound segmented and tracked using the Phasefocus CAT software over 48 hours. b) Motility and c) Mean morphological metrics calculated from the tracked cells.
Conclusion
In this application note we have demonstrated the advanced capabilities of the Phasefocus Livecyte system to image and analyse the wound healing assay. The non-invasive nature of the measurement allows for higher temporal intervals to be imaged and thus a more accurate measure of wound closure over time to be achieved. Further to information regarding collective migration, the high contrast, quantitative images produced, enable multi parametric analysis to be performed at a single cell level. This single-cell analysis directly measures cell migration, while the morphological information extracted, provides both a behavioural and morphological analysis of individual cells during the wound healing process.
Nuo Hai life sciences solutions:


Contact us immediately:
Tel:021-37827858
Http:www.nuohailifescience.com
E-mail:info@nuohailifescience.com





